Using the EDA to teach
Find out how to use the EDA when teaching experimental design
Indicate the strategy for deciding the order of measurements
Information crucial to the internal validity of the experiment is missing. The field ‘measurement order strategy’ is considered compulsory.
Indicate how the order in which individual experimental units are measured will be decided. Examples include randomising, systematically alternating between groups (e.g. measuring one experimental unit from each different group and then a second experimental unit from each different group etc), by experimental group (i.e. measuring all experimental units from one group first, then all experimental units from another group etc).
The order in which you measure animals, experimental units or samples should be randomised if possible to reduce bias.
The allocation section on the EDA website has information on different ways to generate randomisation sequences.
The measurement section on the EDA website has examples of how randomisation and blinding / masking can be used together to reduce bias when taking measurements.
What is an Experimental Design Report?
An Experimental Design Report is a summary of an experiment that a researcher has planned with the Experimental Design Assistant (EDA) online software. The report provides readers with a blueprint of a single experiment, and the minimum information required to allow for an assessment of whether it has been designed rigorously. The report can used as part of ethical review, a funding application or as part of a publication.
Only one sex is being studied in this experiment
You have indicated you are only planning to use one sex in the properties of the animal characteristics node(s). You need to consider if using one sex is appropriate for your experiment, and if so, provide a justification for not using both sexes.
Is using one sex appropriate for your experiment?
The conclusions you can draw from your experiment will be limited by using animals of only one sex. Using male and female animals improves the external validity of an experiment as it enables generalisation of the findings to the whole population. Sex differences exist in disease prevalence, symptoms and progression, as well as in treatment efficacy and side effects. Large scale rodent studies also frequently show treatment effects differ between males and females.
Including both sexes in your experiment is not necessarily the same as specifically looking for sex differences and does not automatically require using more animals.
For more information about taking sex into account when designing experiments, click here.
Justifying not using both sexes
If you are only examining one sex you need to justify this design decision carefully. A lack of evidence for a difference between sexes does not mean a difference does not exist, and so is not an adequate justification for only studying one sex.
The justification for a single-sex experiment can be scientific or practical/logistical. An example scientific justification is that the process only occurs in one sex (e.g. oogenesis, or prostate or ovarian cancer). An example practical/logistical justification is that the experiment involves very rare samples (e.g. conservation studies where access to one sex is more difficult, or studies that include human tissue samples from rare diseases).
If you have already included a justification for not using both sexes you may disregard this message.
Checking if your experiment is sex-inclusive
To check if your experiment is sex-inclusive use the Sex Inclusive Research Framework, a decision tree with supporting guidance for each question. This guidance includes examples of both appropriate and inadequate justifications for single-sex experiments.
Changing the EDA diagram
Including both sexes
Should you decide to include both sexes in your experiment ensure you have an animal characteristics node for each sex. If you have animals with combinations of different characteristics (e.g. different genotypes and different sexes or different ages and different strains), ensure you have an animal characteristics node for each combination (see screenshot below).
Justifying not using both sexes
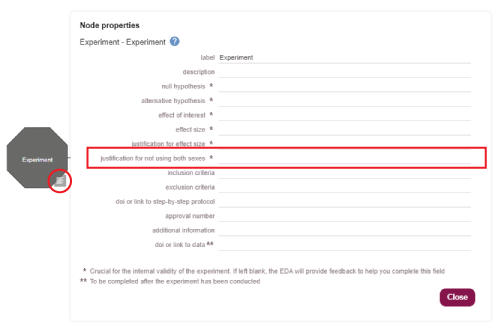
For a study not including both sexes add the justification in the properties of the Experiment node. To open the properties window click on the properties icon on the bottom right of the Experiment node (circled in red in the image below). Add justification information into the field ‘Justification for not using both sexes’ (outlined in red in the image below).
Please note if you modify the diagram the critique will need to be repeated.
Indicate the inclusion and exclusion criteria
Information crucial to the internal validity of the experiment is missing. The following three fields are considered compulsory
In the experiment node:
- Inclusion criteria
- Exclusion criteria
In the analysis node:
- Criteria for excluding data points from the analysis
Inclusion and exclusion criteria define how you will decide the eligibility or disqualification for animals (or experimental units), samples and data once a study has started. It is important to decide these before your experiment begins to prevent you from unconsciously biasing your results by deciding as the experiment progresses.
Inclusion criteria
Inclusion criteria define the prerequisites animals (or experimental units) and samples must meet to be eligible for inclusion in the experiment.
Examples include:
- A tumour must reach a specific minimum size
- Animals must meet a specific training threshold
- Body weight must be within a certain range to be able to undergo a procedure
- A minimum deficit for an induced experimental model to adequately model a specific aspect of a disease.
If you are not setting inclusion criteria for your study explicitly state this (e.g. “no a priori inclusion criteria”).
Exclusion criteria
Exclusion criteria define the criteria that will disqualify animals (or experimental units) and samples from being included or staying in the experiment.
Examples include:
- If any animals develop a motor impairment that would affect a behavioural measurement, they will be removed from the study.
- If any animals develop complications from a surgical procedure, they will be removed from the study.
- Animals reach a humane endpoint, they will be removed from the study and euthanised (e.g. a maximum tumour volume).
- If any samples are damaged during sample collection or preparation the sample will be excluded (e.g. if a histological section is badly damaged during processing and cannot be used).
- If any sample volumes are not large enough to be reliably measured the sample will be excluded from the study (e.g. a blood sample is too small to accurately measure a specific metabolite).
- Any samples that fail to meet prespecified quality control standards or that have unacceptable levels of contamination will not be used.
For criteria to exclude data from the analysis use the field ‘Criteria for excluding data points from the analysis’ in the analysis node.
If losses are anticipated, these should be taken into account when determining the number of animals to include in the study. For example, if your experiment involves a surgical intervention that has a 10% attrition rate, add an extra 10% to your calculated sample size account for expected attrition.
If you are not setting exclusion criteria for your study explicitly state this (e.g. “no a priori exclusion criteria”).
Criteria for excluding data points from the analysis
Criteria that would disqualify data from being included in the analysis should be defined before you start your study to prevent subconscious bias influencing decisions.
Examples include:
- Data values outside a biologically plausible range will not be included in the analysis (e.g. a negative value for body weight).
- Data values resulting from an error during data collection will not be included in the analysis (e.g. someone forgets to switch on equipment for a recording).
- Typos in data values will not be included in the analysis (e.g. if errors are transcribed when copying from the original data source).
- Outliers will be removed using a built-in model in a statistical package (e.g. Grubbs’ method in GraphPad Prism).
If are not setting exclusion criteria for the data in your study explicitly state this (e.g. “no a priori exclusion criteria for data”).
See ARRIVE guidelines item 3b for guidance on responsible data cleaning.
Inclusion and exclusion
How to define criteria for including or excluding animals, experimental units, samples or data points from an experiment
Reed, DR, et al. (2008). Reduced body weight is a common effect of gene knockout in mice. BMC Genet 9:4. doi: 10.1186/1471-2156-9-4
Karp, NA, et al. (2012). The fallacy of ratio correction to address confounding factors. Lab Anim 46(3):245-52. doi: 10.1258/la.2012.012003
Altman, DG and Bland, JM (2005). Treatment allocation by minimisation. BMJ 330(7495):843. doi: 10.1136/bmj.330.7495.843


